Anodic oxidation is a process in which the current anode flows through the metal, the oxidation liquid flows as the cathode, the positive elements on the metal surface react with the negative electrode, and a certain thickness of oxide film is formed on the metal surface after a period of reaction.
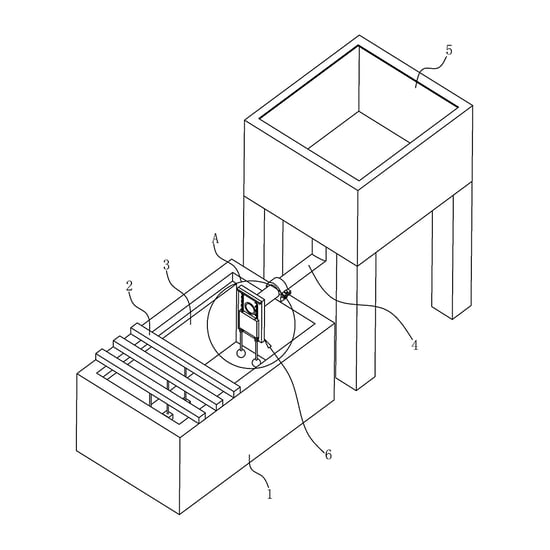
The positive elements on the metal surface react with the negative electrode.
By adding pigments to the oxidation liquid, various colors of oxygenated layers can be formed.

Anodizing can cooperate with sandblasting process to form sandblasting layers of various colors on the surface of parts.
Polishing in a broad sense refers to the use of metal, non-metal, chemical, electrical and other media to contact on the rough surface and make the surface flat and smooth through friction to achieve the effect of removing concave and convex particles.

1. Mechanical polishing. Through high-speed friction, the rust layer and fine defects on the workpiece surface are polished smooth. Mechanical polishing is suitable for surface polishing of large quantities of workpieces and can be part of the assembly line process.

2. Hand polishing. Manual polishing is generally used to polish the contact surface (appearance surface) of clocks, ornaments, abrasives, and machine tools that need mirror polishing. The demand for workpiece can be realized through manual experience and customized polishing equipment and polishing media.
Electro polishing, also known as electropolishing, is a method that uses the electrochemical dissolution phenomenon of the anode in the electrolytic cell to selectively dissolve the micro convex part of the anode to form a smooth surface. It is a complex anodic oxidation process, accompanied by dissolution and oxidation of the workpiece surface, but it is different from anodic oxidation.


Electroplating is the process of covering a metal layer on the surface of the workpiece. After electroplating, the surface of the workpiece will change its wear resistance, conductivity, reflectance, and corrosion resistance (copper sulfate, etc.) and improve its appearance.

Make the workpiece conductive

Conductive or insulating

Improve corrosion resistance
The electrophoretic coating is a coating method that utilizes an electric field to make particles such as pigments and resins suspended in the electrophoretic solution migrate directionally and deposit on the substrate surface of one of the electrodes. The electrophoresis process is safe and environmentally friendly. (Complete coating is used for complex workpiece.)

Fine coating is efficient.

High utilization makes it widely used.
Metal blackening is a common metal surface treatment process. The purpose of blackening is to prevent rust on the metal surface. There are two kinds of metal blackening: alkaline heating blackening and normal temperature blackening. The problem of checking the antirust effect may occur due to process problems in normal temperature blackening.


High pressure airless spraying process is adopted in powder coating. The high pressure airless spraying opertes on the premise of pressurizing the coating to 210 kg/c㎡, atomizing the coating into fine particles through the nozzle, and then directly spraying it onto the surface of the coated object, which is a promising high-efficiency spraying method.

High-pressure airless spraying is not only suitable for spraying ordinary paint, but also especially suitable for spraying high viscosity paint.

When the high-pressure coating leaves the nozzle and reaches the atmosphere, it will immediately expand violently.

Unlike air spraying, it uses a high-pressure pump to pressurize the paint to about 15MPa, and then sprays it through a special nozzle hole.
In the plating tank containing zinc plating solution, the cleaned and specially pretreated parts to be plated are used as the cathode, and the anode is made of plated metal. The two poles are respectively connected with the positive and negative electrodes of the DC power supply. The zinc plating solution is composed of an aqueous solution containing a compound of a plating metal, a conductive salt, a buffer, a pH regulator, and an additive. After being electrified, the metal ions in the zinc plating solution move to the cathode under the action of the potential difference to form a coating. The metal of the anode forms metal ions into the zinc plating solution to maintain the concentration of the metal ions to be plated.

In some cases, such as chromium plating, it is an insoluble anode made of lead and leads antimony alloy, which only serves to transfer electrons and conduct current.
The chromium ion concentration in the electrolyte needs to be maintained by regularly adding chromium compounds to the plating solution.

During galvanizing, the quality of the anode material, the composition of the galvanizing solution, the temperature, the current density, the energizing time, the stirring strength, the precipitated impurities, the power waveform, etc. will all affect the quality of the coating.
The pattern to be printed is first made into a steel plate (or copper, thermoplastic) intaglio by film etching, and then a curved surface pad printing head made of silicone rubber material is used to dip the ink on the intaglio plate into the surface of the pad printing head, and then press it on the surface of the desired object to print the text and pattern.


